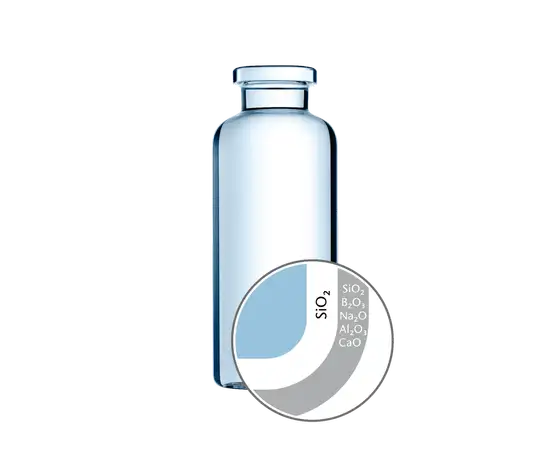
Product Variants of Glass Vials
SCHOTT produces a broad range of specialist vials for specific applications. From SCHOTT Type I plus® for reduced drug-container action to EVERIC® smooth for friction-free movement on filling lines, our vials are manufactured and packed in environmentally controlled areas, and conform to all major standards.
EVERIC® pure
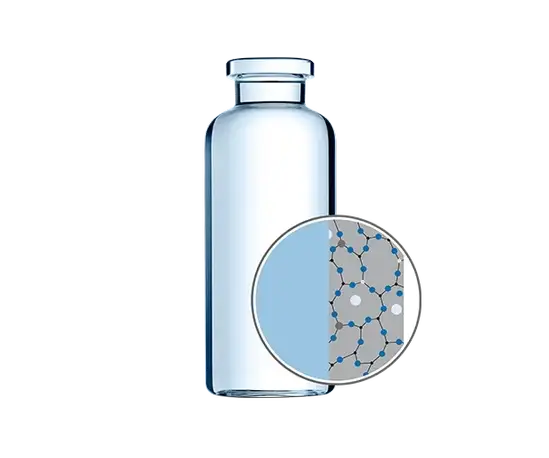
More than two thirds of all drugs are biologics, with a large number filled in dosages under 1 ml. In such low-fill scenarios, the high ratio of container surface to drug volume leads to a proportional increase in exposure to leachables, which may potentially harm the drug.
CHARACTERISTICS
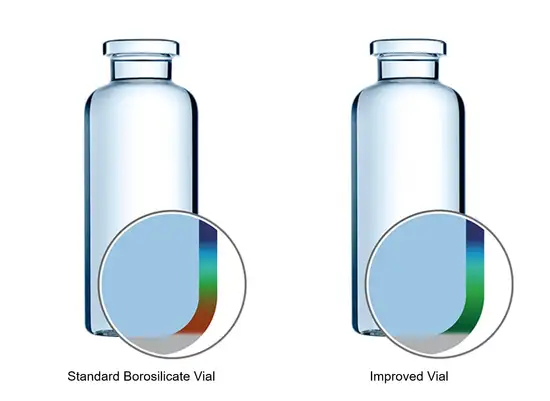
To meet these demands, SCHOTT has developed EVERIC® pure for sensitive drugs and drugs with low filling volumes. The new vials ensure drug stability by using an improved borosilicate glass tubing – FIOLAX® CHR (controlled hydrolytic resistance) – which has a higher chemical stability without any change in glass composition. Thanks to existing regulatory validation, companies are able to replace conventional tubular Type-I glass vials for existing drugs with EVERIC® pure without costly re-registration.
ADVANTAGES
- Unmatched drug stability for low-filling volumes.
- Minimized delamination risk.
- Patented SCHOTT manufacturing technology and SCHOTT Quicktest for validation and product release, supplied with certificate of compliance (COC).
- Applicable to all products already registered – no re-registration required.
EVERIC® care
Glass leachables can cause stability issues for complex and sensitive injectables solutions, as they may appear in biologics. These occur especially for drugs formulated in the high pH range which represent a challenge for current packaging solutions. This product offers a new and innovative single-layer PICVD barrier coating with hydrophobic surface inside the EVERIC® pure glass vial. In addition, the hydrophobic surface can support the residual emptying of the vial.
CHARACTERISTICS
EVERIC® care is the only barrier coating for high pH fillings with hydrophobic surface combining all advantages of the Type I middle borosilicate glass container and the well-known 1-layer PICVD coating of SCHOTT.
The interior coating combines the hydrophobic behavior known from polymeric containers withstanding higher pH ranges and the typical glass vial with its known advantages for production, inspection and storage.
ADVANTAGES
- Barrier function ensuring low leachable levels especially in the alkaline pH range
- A proven technology has been used by choosing the patented PICVD coating technology of SCHOTT.
- Like for the whole EVERIC® family an additional “release criteria” has been specifically developed for high pH value applications
EVERIC® strong
SCHOTT used computer simulation to improve formation and geometry at the vial’s handling and contact points in the heel and shoulder area to create EVERIC® strong.
CHARACTERISTICS
SCHOTT used computer simulation to improve formation and geometry at the vial’s handling and contact points in the heel and shoulder area to create EVERIC® strong. As a result, these vials can better withstand side compression and axial load during filling and transportation, reducing the risk of glass breakage.
ADVANTAGES
- Eliminates inherent stress.
- A flawless, optimized process at all handling and contact points.
- Improved forming process, resulting in improved strength in side compression and axial load.
- Unmatched optimized strength within existing ISO tolerances and an unchanged glass matrix.
EVERIC® smooth
Direct vial-to-vial contact on conventional filling lines leads to various cosmetic defects. These scratches and abrasions increase the number of final rejects and reduce the container’s strength, which may lead to glass breakage. The consequences: disruption and increased manufacturing costs.
CHARACTERISTICS
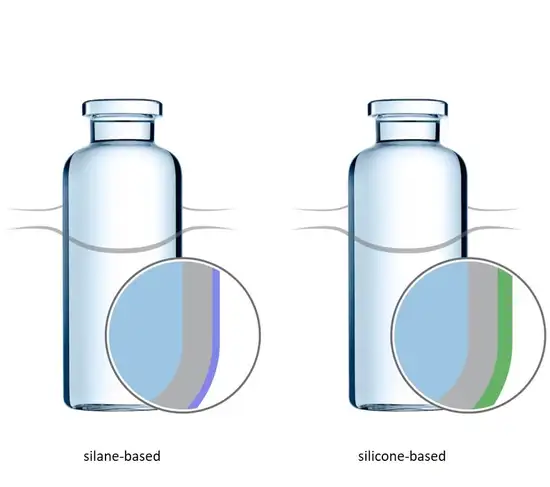
To ensure a smooth container flow and a reduction of cosmetic defects, SCHOTT offers a vial with an outer coating that features a 56% reduced coefficient of friction: EVERIC® smooth. Customers can choose between two coatings, which both show a hydrophobic surface behaviour: silicone- or silane-based. The silicone-based coating is applied via dip-coating and adhered to the glass surface by heat treatment. The silane-based coating is nano-particle-free and attained by covering the container with a covalent bond molecular layer.
ADVANTAGES
- Reduction of cosmetic defects due to protection of outer surface.
- Maintained container strength: Reduced risk and efficient line performance.
- Yield increase by smooth container flow.
Type I plus®
Protein adsorption and leaching from primary glass packaging are the root causes of reduced shelf life in sensitive formulations. Metal ion leaching from type I glass leads to a pH shift in water for injectable or unbuffered solutions, which can decrease the stability or activity of biopharmaceuticals.
CHARACTERISTICS
SCHOTT Type I plus® vials have a silicon dioxide coating on the inside surface that has outstanding barrier properties. This reduces the interaction between drug and container surface to a bare minimum, providing a superior packaging solution for sensitive ingredients.
YOUR ADVANTAGES
- Increased shelf-life stability for sensitive drug formulations.
- Minimized adsorption of proteins in liquid formulations – also prior to lyophilization.
- Minimized adsorption of radioactive molecules.
SCHOTT TopLyo®
Because of a need to accelerate time-to-market cycles and increase the shelf-life stability of biopharmaceuticals, there’s a keen interest in robust and cost-efficient lyophilization. A streamlined lyo cake is crucial for improved automation of inspectability and fundamental to patient safety.
CHARACTERISTICS
Thanks to their hydrophobic coating, SCHOTT TopLyo® vials demonstrate less fogging during the lyophilization process and less disruption of the lyo cake after, in addition to reduced residual volumes and higher dosing accuracy after reconstitution.
ADVANTAGES
- Cost reduction due to decreased overfilling – given lower residual volume.
- Prevention of lyo cake disruption and sidewall fogging for improved automated inspection.
- Lyo cake stability during transportation.
- Reduced protein aggregation compared to siliconized vials.

Florence Buscke
Sr. Global Product Manager